|
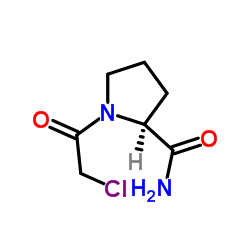
![NVP DPP 728 Structure]()
NVP DPP 728
CAS#:247016-69-9
|
|
Literature: Villhauer, Edwin B.; Brinkman, John A.; Naderi, Goli B.; Dunning, Beth E.; Mangold, Bonnie L.; Mone, Manisha D.; Russell, Mary E.; Weldon, Stephen C.; Hughes, Thomas E. Journal of Medicinal Chemistry, 2002 , vol. 45, # 12 p. 2362 - 2365
|
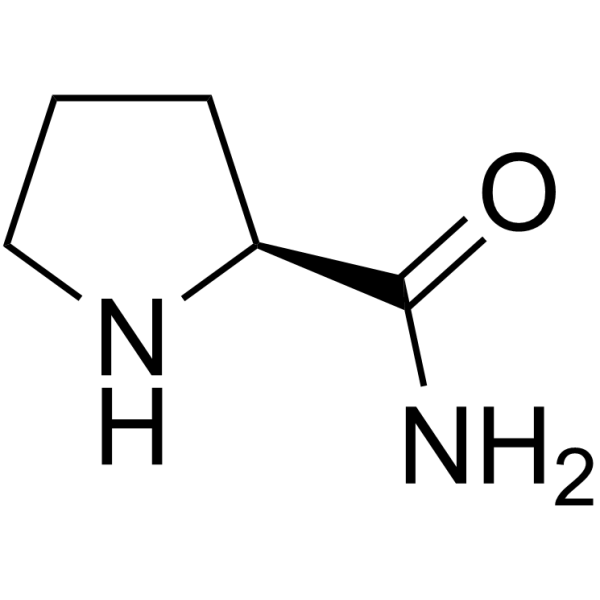
![NVP DPP 728 Structure]()
NVP DPP 728
CAS#:247016-69-9
|
|
Literature: Villhauer, Edwin B.; Brinkman, John A.; Naderi, Goli B.; Burkey, Bryan F.; Dunning, Beth E.; Prasad, Kapa; Mangold, Bonnie L.; Russell, Mary E.; Hughes, Thomas E. Journal of Medicinal Chemistry, 2003 , vol. 46, # 13 p. 2774 - 2789
|
![NVP DPP 728 Structure]()
NVP DPP 728
CAS#:247016-69-9
|
|
Literature: Villhauer, Edwin B.; Brinkman, John A.; Naderi, Goli B.; Burkey, Bryan F.; Dunning, Beth E.; Prasad, Kapa; Mangold, Bonnie L.; Russell, Mary E.; Hughes, Thomas E. Journal of Medicinal Chemistry, 2003 , vol. 46, # 13 p. 2774 - 2789
|
![NVP DPP 728 Structure]()
NVP DPP 728
CAS#:247016-69-9
|
|
Literature: Willand, Nicolas; Joossens, Jurgen; Gesquiere, Jean-Claude; Tartar, Andre L; Evans, D.Michael; Roe, Michael B Tetrahedron, 2002 , vol. 58, # 28 p. 5741 - 5746
|
![NVP DPP 728 Structure]()
NVP DPP 728
CAS#:247016-69-9
|
|
Literature: Willand, Nicolas; Joossens, Jurgen; Gesquiere, Jean-Claude; Tartar, Andre L; Evans, D.Michael; Roe, Michael B Tetrahedron, 2002 , vol. 58, # 28 p. 5741 - 5746
|
![NVP DPP 728 Structure]()
NVP DPP 728
CAS#:247016-69-9
|
|
Literature: Villhauer, Edwin B.; Brinkman, John A.; Naderi, Goli B.; Dunning, Beth E.; Mangold, Bonnie L.; Mone, Manisha D.; Russell, Mary E.; Weldon, Stephen C.; Hughes, Thomas E. Journal of Medicinal Chemistry, 2002 , vol. 45, # 12 p. 2362 - 2365
|

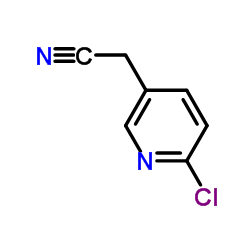
![6-[(2-AMINOETHYL)AMINO]NICOTINONITRILE structure](https://www.chemsrc.com/caspic/498/202460-48-8.png)