Cryo-EM structure of the Blastochloris viridis LH1–RC complex at 2.9 Å
Pu Qian, C. Alistair Siebert, Peiyi Wang, Daniel P. Canniffe, C. Neil Hunter
文献索引:10.1038/s41586-018-0014-5
全文:HTML全文
摘要
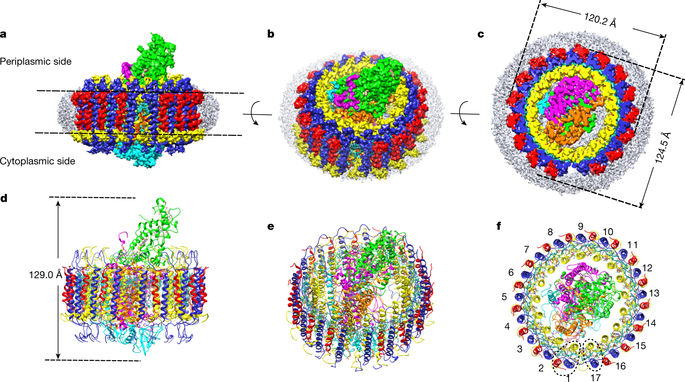
The light-harvesting 1–reaction centre (LH1–RC) complex is a key functional component of bacterial photosynthesis. Here we present a 2.9 Å resolution cryo-electron microscopy structure of the bacteriochlorophyll b-based LH1–RC complex from Blastochloris viridis that reveals the structural basis for absorption of infrared light and the molecular mechanism of quinone migration across the LH1 complex. The triple-ring LH1 complex comprises a circular array of 17 β-polypeptides sandwiched between 17 α- and 16 γ-polypeptides. Tight packing of the γ-apoproteins between β-polypeptides collectively interlocks and stabilizes the LH1 structure; this, together with the short Mg–Mg distances of bacteriochlorophyll b pairs, contributes to the large redshift of bacteriochlorophyll b absorption. The ‘missing’ 17th γ-polypeptide creates a pore in the LH1 ring, and an adjacent binding pocket provides a folding template for a quinone, Q P, which adopts a compact, export-ready conformation before passage through the pore and eventual diffusion to the cytochrome bc1 complex.
|
Vms1 and ANKZF1 peptidyl-tRNA hydrolases release nascent cha...
2018-04-09 [10.1038/s41586-018-0022-5] |
|
Fatal swine acute diarrhoea syndrome caused by an HKU2-relat...
2018-04-04 [10.1038/s41586-018-0010-9] |
|
Crystal structures of the gastric proton pump
2018-04-04 [10.1038/s41586-018-0003-8] |
|
The evolutionary history of vertebrate RNA viruses
2018-04-04 [10.1038/s41586-018-0012-7] |
|
Accelerated increase in plant species richness on mountain s...
2018-04-04 [10.1038/s41586-018-0005-6] |