Structure of photosynthetic LH1–RC supercomplex at 1.9 Å resolution
Long-Jiang Yu, Michihiro Suga, Zheng-Yu Wang-Otomo, Jian-Ren Shen
文献索引:10.1038/s41586-018-0002-9
全文:HTML全文
摘要
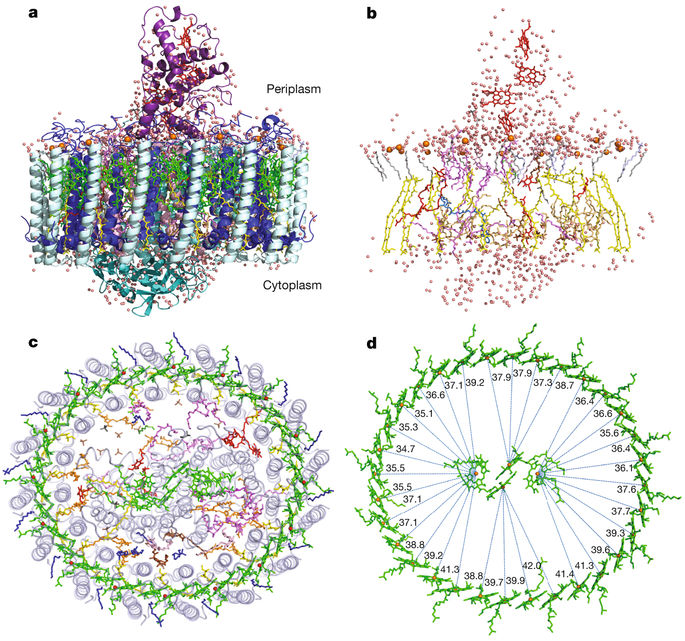
Light-harvesting complex 1 (LH1) and the reaction centre (RC) form a membrane-protein supercomplex that performs the primary reactions of photosynthesis in purple photosynthetic bacteria. The structure of the LH1–RC complex can provide information on the arrangement of protein subunits and cofactors; however, so far it has been resolved only at a relatively low resolution. Here we report the crystal structure of the calcium-ion-bound LH1–RC supercomplex of Thermochromatium tepidum at a resolution of 1.9 Å. This atomic-resolution structure revealed several new features about the organization of protein subunits and cofactors. We describe the loop regions of RC in their intact states, the interaction of these loop regions with the LH1 subunits, the exchange route for the bound quinone QB with free quinone molecules, the transport of free quinones between the inside and outside of the LH1 ring structure, and the detailed calcium-ion-binding environment. This structure provides a solid basis for the detailed examination of the light reactions that occur during bacterial photosynthesis.
|
Vms1 and ANKZF1 peptidyl-tRNA hydrolases release nascent cha...
2018-04-09 [10.1038/s41586-018-0022-5] |
|
Fatal swine acute diarrhoea syndrome caused by an HKU2-relat...
2018-04-04 [10.1038/s41586-018-0010-9] |
|
Crystal structures of the gastric proton pump
2018-04-04 [10.1038/s41586-018-0003-8] |
|
The evolutionary history of vertebrate RNA viruses
2018-04-04 [10.1038/s41586-018-0012-7] |
|
Accelerated increase in plant species richness on mountain s...
2018-04-04 [10.1038/s41586-018-0005-6] |