Carbon and hydrogen isotope fractionation of phthalate esters during degradation by sulfate and hydroxyl radicals
Dan Zhang, Langping Wu, Jun Yao, Hartmut Herrmann, Hans-Hermann Richnow
文献索引:10.1016/j.cej.2018.04.047
全文:HTML全文
摘要
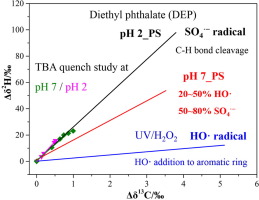
This study investigated 13C and 2H isotope fractionation associated with oxidation of three phthalate esters (PAEs) by radical species, including sulfate radical (SO4·−) induced by heat-activated persulfate (PS) and hydroxyl radical (HO·) induced by UV/H2O2. For persulfate oxidation at pH = 2 and pH = 7, similar carbon isotope fractionation (εC) but distinct hydrogen isotope enrichment factors (εH) were observed. The UV/H2O2 reaction of three PAEs showed smaller εH values in comparison with persulfate oxidation. The correlation of 2H and 13C fractionation (Λ) allows to distinguish the persulfate oxidation (25.7±2.6) and UV/H2O2 oxidation (2.4±0.2) of diethyl phthalate (DEP) highlighting the potential of compound-specific stable isotope analysis (CSIA) to characterize chemical oxidation mechanism of PAEs. Moreover, study of radical quenching and CSIA were combined to explore the dominant radical species during persulfate oxidation of DEP. SO4·− was found to be the predominant radical at pH = 2. Both SO4·− and HO· contributed to DEP degradation at pH = 7 and HO· was estimated to have a contribution of 21-63% according to dual C-H isotope fractionation values. Carbon and hydrogen apparent kinetic isotope effects (AKIEs) (13C-AKIE =1.017, 2H-AKIE =2.41) obtained from dominating sulfate radical reaction of DEP both supported the hypothesis of C-H bond cleavage. Thus, carbon and hydrogen isotope enrichment factors clearly distinguish the different reaction mechanisms and hence, are a promising approach to improve understanding of radical species reaction pathways for chemical oxidation of PAEs.
|
Long-term activity of a CuO/SBA-15 type SOx adsorbent: impac...
2018-04-11 [10.1016/j.cej.2018.04.066] |
|
Chemical, Microbial and Toxicological Assessment of Wastewat...
2018-04-11 [10.1016/j.cej.2018.04.037] |
|
Kinetics of coupling cracking of butene and pentene on modif...
2018-04-10 [10.1016/j.cej.2018.04.061] |
|
Hierarchically Porous Carbon Derived from Metal-Organic Fram...
2018-04-09 [10.1016/j.cej.2018.04.051] |
|
Adsorption and Desorption of U(VI) on Different-size Graphen...
2018-04-09 [10.1016/j.cej.2018.04.050] |