Complexation of trans- and cis-resveratrol with bovine serum albumin, β-lactoglobulin or α-lactalbumin
Hao Cheng, Zheng Fang, Wusigale, Amr M. Bakry, Yantao Chen, Li Liang
文献索引:10.1016/j.foodhyd.2018.02.037
全文:HTML全文
摘要
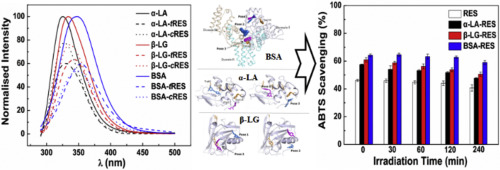
Clarification of the mechanism of protein-ligand interaction is important for the encapsulation of bioactive components. Trans-resveratrol is labile to convert to cis-isomer during fermentation and storage, but the cis-isomer interaction with proteins has not yet gained as much attention as that of trans-isomer. The influence of resveratrol's isomerization on interaction with ligand-binding proteins was investigated here. Cis-resveratrol exhibited higher affinity for bovine serum albumin than trans-isomer, but the isomerization to cis-isomer decreased the polyphenol affinity for α-lactalbumin and β-lactoglobulin. For each protein, trans- and cis-resveratrol shared the same optimal docking site but showed clear diversity in the second best site. Protein ternary complex could be formed when trans-/cis-resveratrol were added in the sequence. Moreover, photostability of resveratrol and antioxidant activity of the polyphenol-protein mixtures were also discussed. The results gathered here should provide further insight into protein-polyphenol interactions and be useful for the development of protein-based carriers for the polyphenols.
|
Physicochemical, textural, rheological and microstructural p...
2018-04-03 [10.1016/j.foodhyd.2018.04.008] |
|
High Internal Phase Emulsions Stabilized by Starch Nanocryst...
2018-04-03 [10.1016/j.foodhyd.2018.04.006] |
|
Effect of pH and salts on microstructure and viscoelastic pr...
2018-04-03 [10.1016/j.foodhyd.2018.04.005] |
|
Incidence of milling energy on dry-milling attributes of ric...
2018-04-03 [10.1016/j.foodhyd.2018.03.051] |
|
Stabilization of oil continuous emulsions with colloidal par...
2018-04-03 [10.1016/j.foodhyd.2018.04.004] |