Sequential Ytterbium(III) Triflate Catalyzed One‐Pot Three‐Component Thia‐Michael Addition
Hsin‐Yi Huang; Chien‐Fu Liang
文献索引:10.1002/ajoc.201800087
全文:HTML全文
摘要
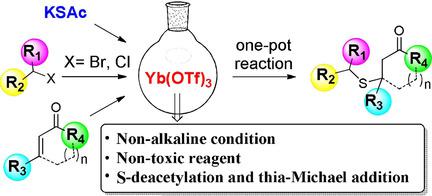
A ytterbium(III) trifluoromethanesulfonate catalyzed one‐pot three‐component thia‐Michael addition reaction of thiolate salts, organic halides, and α,β‐unsaturated compounds has been developed for the formation of carbon‐sulfur bonds. In this study, potassium thioacetate serves as the sulfur source in a nucleophilic substitution reaction, which is followed by a Yb(OTf)3‐catalyzed (OTf=trifluoromethanesulfonate) S‐deacetylation and subsequent thia‐Michael addition reaction. Three important pharmaceutical derivatives were successfully prepared by using the intramolecular version of this one‐pot thia‐Michael addition reaction. The advantages of this YbIII‐catalyzed process include its operational simplicity, its capacity towards the preparation of structurally diverse products in 40–93 % yield, its use of relatively low toxic or nontoxic reagents, and its catalyst loading of only 1–2 mol %.
|
A Copper‐Catalyzed Domino Reaction of Alkynyl Bromides and O...
2018-04-14 [10.1002/ajoc.201800154] |
|
Recent Progress in Using Pyrene‐4,5‐diketones and Pyrene‐4,5...
2018-04-10 [10.1002/ajoc.201800039] |
|
Chelation‐assisted β‐selective direct C‐H bond arylation of ...
2018-04-06 [10.1002/ajoc.201800160] |
|
Radical Reaction for the Synthesis of Thiopyrans under Photo...
2018-04-06 [10.1002/ajoc.201800159] |
|
Light‐Induced Cyclization of A [c2]Daisy‐Chain Rotaxane to F...
2018-04-06 [10.1002/ajoc.201800114] |