Reductive Defluorination of Branched Per- and Polyfluoroalkyl Substances with Cobalt Complex Catalysts
Jinyong Liu, Daniel J. Van Hoomissen, Tianchi Liu, Andrew Maizel, Xiangchen Huo, Seth R. Fernández, Changxu Ren, Xin Xiao, Yida Fang, Charles E. Schaefer, Christopher P. Higgins, Shubham Vyas, Timothy J. Strathmann
文献索引:10.1021/acs.estlett.8b00122
全文:HTML全文
摘要
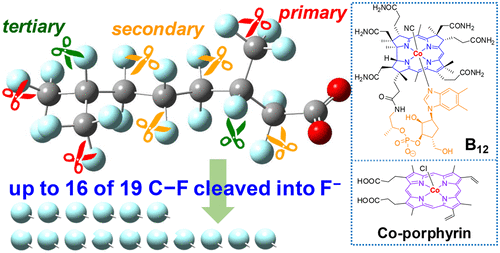
This study investigates structure–reactivity relationships within branched per- and polyfluoroalkyl substances (PFASs) undergoing cobalt-catalyzed reductive defluorination reactions. Experimental results and theoretical calculations reveal correlations among the extent of PFAS defluorination, the local C–F bonding environment, and calculated bond dissociation energies (BDEs). In general, BDEs increase in the following order: tertiary C–F bonds < secondary C–F bonds < primary C–F bonds. A tertiary C–F bond adjacent to three fluorinated carbons (or two fluorinated carbons and one carboxyl group) has a relatively low BDE that permits an initial defluorination to occur. Both a biogenic cobalt–corrin complex (B12) and an artificial cobalt–porphyrin complex (Co-PP) are found to catalytically defluorinate multiple C–F bonds in selected PFASs. In general, Co-PP exhibits an initial rate of defluorination that is higher than that of B12. Neither complex induced significant defluorination in linear perfluorooctanoic acid (PFOA; no tertiary C–F bond) or a perfluoroalkyl ether carboxylic acid (tertiary C–F BDEs too high). These results open new lines of research, including (1) designing branched PFASs and cobalt complexes that promote complete defluorination of PFASs in natural and engineered systems and (2) evaluating potential impacts of branched PFASs in biological systems where B12 is present.
|
Modeling Secondary Organic Aerosol Production from Photosens...
2018-04-06 [10.1021/acs.estlett.8b00101] |
|
Kaolin Alleviates Graphene Oxide Toxicity
2018-04-06 [10.1021/acs.estlett.8b00135] |
|
Estimating the Transfer Range of Plasmids Encoding Antimicro...
2018-04-05 [10.1021/acs.estlett.8b00105] |
|
Identification of Chain Scission Products Released to Water ...
2018-04-04 [10.1021/acs.estlett.8b00119] |
|
Biotransformation of AFFF Component 6:2 Fluorotelomer Thioet...
2018-04-04 [10.1021/acs.estlett.8b00148] |