Fluorinated GluN2B Receptor Antagonists with a 3‐Benzazepine Scaffold Designed for PET Studies
Marina Szermerski; Frederik Börgel; Dirk Schepmann; Ahmed Haider; Thomas Betzel; Simon M. Ametamey; Bernhard Wünsch
文献索引:10.1002/cmdc.201700819
全文:HTML全文
摘要
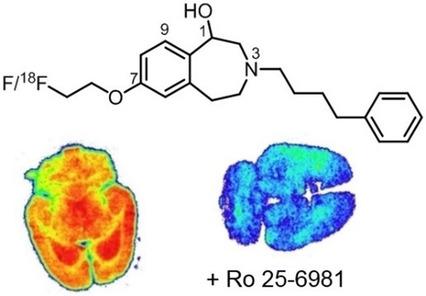
To analyze the N‐methyl‐d‐aspartate (NMDA) receptor distribution in the central nervous system, fluorinated ligands that selectively address the ifenprodil binding site of GluN2B‐subunit‐containing NMDA receptors were developed. Various strategies to introduce a fluorine atom into the potent GluN2B ligand 2 (3‐(4‐phenylbutyl)‐2,3,4,5‐tetrahydro‐1H‐3‐benzazepin‐1,7‐diol) were pursued, including replacement of the benzylic OH moiety with a fluorine atom (13) and introduction of fluoroethoxy moieties at various positions (14 (7‐position), 17 (9‐position), 18a–c (1‐position)). With respect to GluN2B affinity and selectivity over related receptors, the fluoroethoxy derivatives 14 and 18a are the most promising ligands. Radiosynthesis of fluoroethoxy derivative [18F]14 was performed by nucleophilic substitution of the phenol 2 with 2‐[18F]fluoroethyl tosylate. On rat brain slices the fluorinated PET tracer [18F]14 accumulated in regions with high density of NMDA receptors containing GluN2B subunits. The bound radioactivity could not be replaced by (S)‐glutamate. However, the GluN2B ligands eliprodil, Ro 25‐6981, and the non‐labeled 3‐benzazepine 14 were able to abolish the specific binding of [18F]14.
|
Identifying Small‐Molecule Binding Sites for Epigenetic Prot...
2018-04-17 [10.1002/cmdc.201800030] |
|
Synthesis, Pharmacological Evaluation, and Docking Studies o...
2018-04-16 [10.1002/cmdc.201800152] |
|
Discovery of Benzimidazole–Quinolone Hybrids as New Cleaving...
2018-04-16 [10.1002/cmdc.201700739] |
|
Transthyretin Mimetics as Anti‐β‐Amyloid Agents: A Compariso...
2018-04-16 [10.1002/cmdc.201800031] |
|
Antibody Epitope of Human α‐Galactosidase A Revealed by Affi...
2018-04-16 [10.1002/cmdc.201800094] |