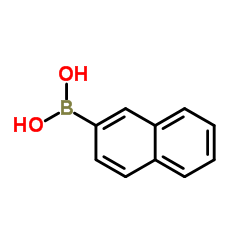
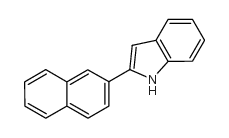
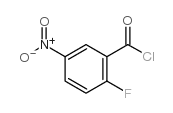
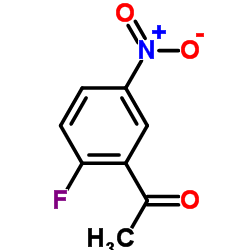
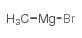|
~96% |
|
~67% |
|
~% |
|
~77% |
|
~% |
|
~81% |
|
~85% |
|
~77% |
|
~73% |
|
~97% |
|
~93% |
|
~% |
|
~86% |
|
~81% |
|
~75% |
|
~79% |
|
~83% |
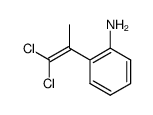
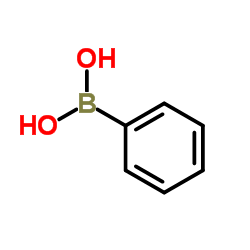
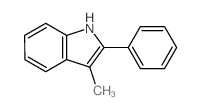
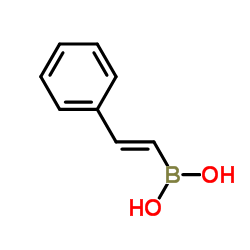
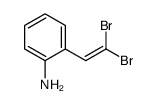

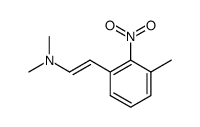
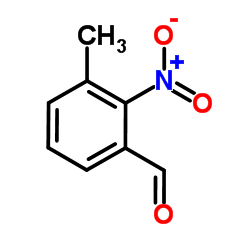

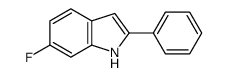
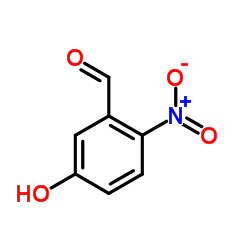
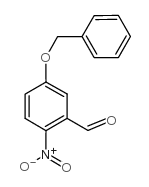
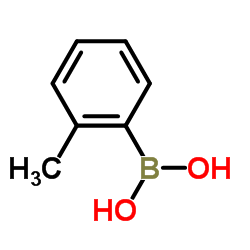

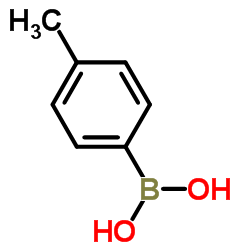
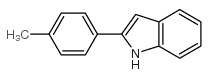
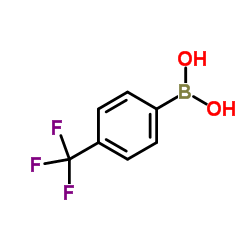
![2-[4-(Trifluoromethyl)phenyl]-1H-indole结构式](https://image.chemsrc.com/caspic/492/243971-02-0.png)
![N-[2-(2,2-dibromovinyl)-phenyl]-acetamide结构式](https://image.chemsrc.com/caspic/058/693794-61-5.png)
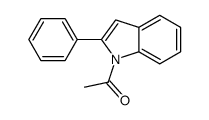
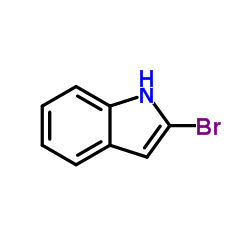

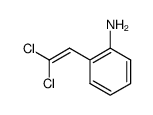
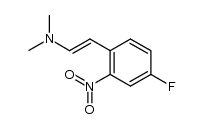
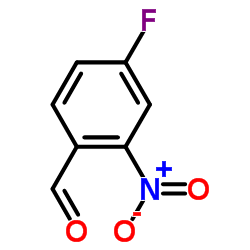

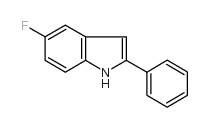
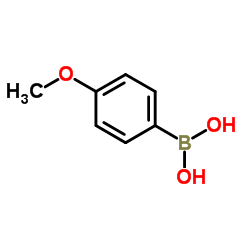
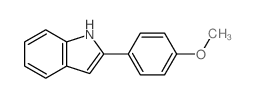
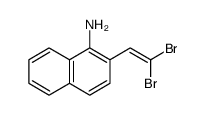
![2-phenyl-1H-benzo[g]indole结构式](https://image.chemsrc.com/caspic/329/33555-17-8.png)