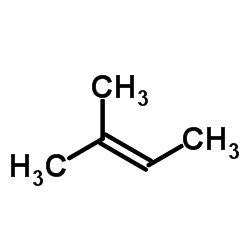
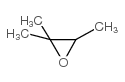
|
~41% |
|
~94% |
|
~85% |
|
~78% |
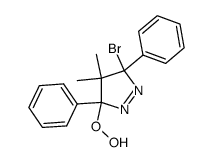
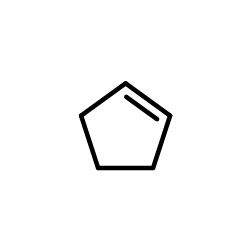

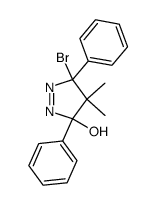
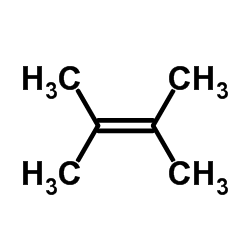
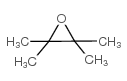
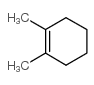
![1,6-dimethyl-7-oxabicyclo[4.1.0]heptane结构式](https://image.chemsrc.com/caspic/351/17612-36-1.png)