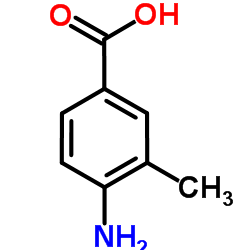
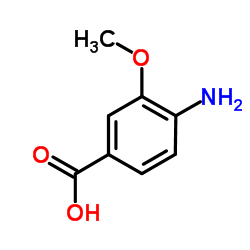
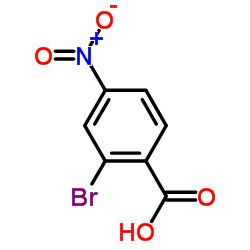
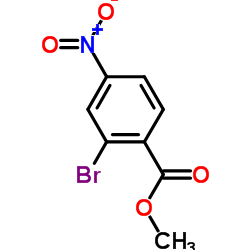
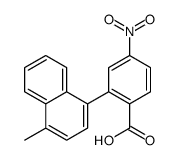
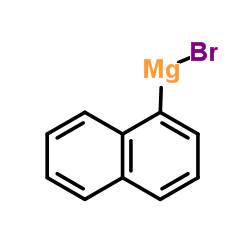
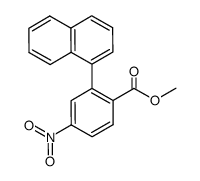
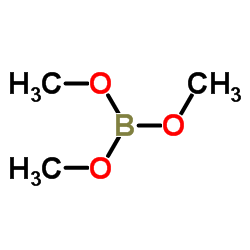
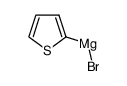
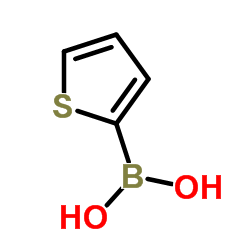
|
~60% |
|
~92% |
|
~89% |
|
~91% |
|
~% |
|
~% |
|
~99% |
|
~66% |