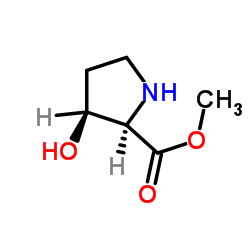
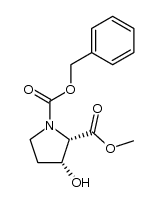
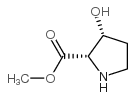
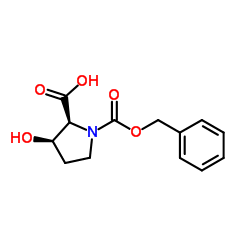
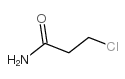
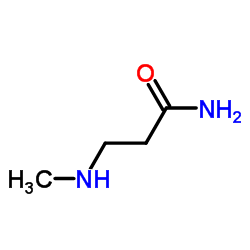
|
~% |
|
~% |
|
~66% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~51% |
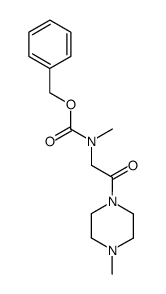
![N-甲基-n-[2-(4-甲基哌嗪-1-基)-2-氧代乙基]胺结构式](https://image.chemsrc.com/caspic/125/166187-00-4.png)


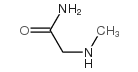
![(2S,3S)-1-[(苄氧基)羰基]-3-羟基吡咯烷-2-羧酸结构式](https://image.chemsrc.com/caspic/097/62182-54-1.png)
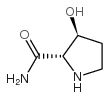
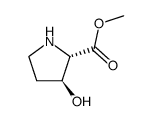
![(2R,3S)-1-[(苄氧基)羰基]-3-羟基吡咯烷-2-羧酸结构式](https://image.chemsrc.com/caspic/182/174389-11-8.png)