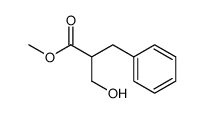
|
~% |
|
~% |
|
~% |
|
~% |
|
~75% |
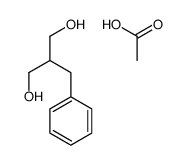
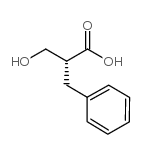
![Benzenepropanoic acid,-alpha--[(acetyloxy)methyl]- (9CI)结构式](https://image.chemsrc.com/caspic/433/187610-68-0.png)