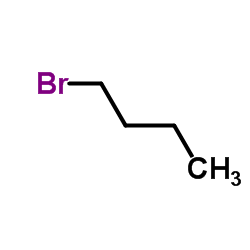
|
~% |
|
~% |
|
~% |
|
~85% |
|
~58% |




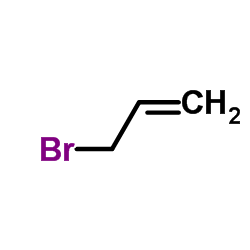
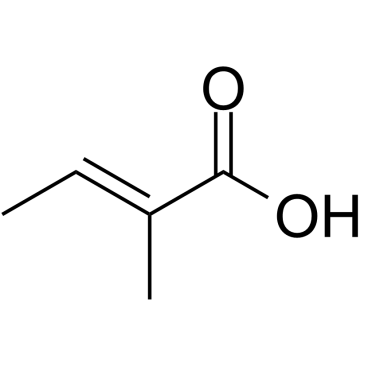

![(4E)-4-[[4-(dimethylamino)anilino]methylidene]-2-phenyl-1,3-oxazol-5-one结构式](https://image.chemsrc.com/caspic/437/5662-80-6.png)