|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![(2R,4a'R,5R,6'S,7'S,8'R,8a'R)-7'-(benzyloxy)-5-(((tert-butyldiphenylsilyl)oxy)methyl)-8'-(methoxymethoxy)octahydro-3H,3'H-spiro[furan-2,2'-pyrano[3,2-b]pyran]-6'-carbaldehyde结构式](https://image.chemsrc.com/caspic/269/104307-01-9.png)
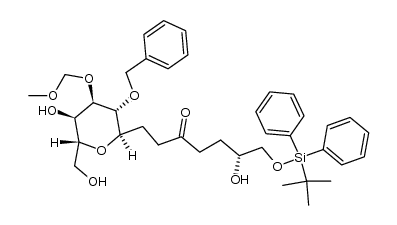
![(6R)-1-((4aR,6R,7R,8S,8aR)-7-(benzyloxy)-8-(methoxymethoxy)-2-phenylhexahydropyrano[3,2-d][1,3]dioxin-6-yl)-7-((tert-butyldiphenylsilyl)oxy)-6-(1-ethoxyethoxy)heptan-3-one结构式](https://image.chemsrc.com/caspic/494/104306-97-0.png)
![(((2R,4a'R,5R,6'R,7'R,8'R,8a'R)-7'-(benzyloxy)-8'-(methoxymethoxy)-6'-((trityloxy)methyl)octahydro-3H,3'H-spiro[furan-2,2'-pyrano[3,2-b]pyran]-5-yl)methoxy)(tert-butyl)diphenylsilane结构式](https://image.chemsrc.com/caspic/307/104307-00-8.png)
![((2R,4a'R,5R,6'R,7'R,8'R,8a'R)-7'-(benzyloxy)-5-(((tert-butyldiphenylsilyl)oxy)methyl)-8'-(methoxymethoxy)octahydro-3H,3'H-spiro[furan-2,2'-pyrano[3,2-b]pyran]-6'-yl)methanol结构式](https://image.chemsrc.com/caspic/162/109413-94-7.png)
![(2R,4a'R,5R,6'R,7'R,8'R,8a'R)-5-(((tert-butyldiphenylsilyl)oxy)methyl)-6'-(hydroxymethyl)-8'-(methoxymethoxy)octahydro-3H,3'H-spiro[furan-2,2'-pyrano[3,2-b]pyran]-7'-ol结构式](https://image.chemsrc.com/caspic/418/104306-99-2.png)