|
~28% |
|
~42% |
|
~90% |
|
~21%
详细
|
|
~85% |

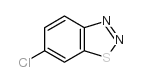
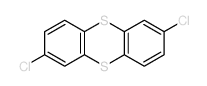

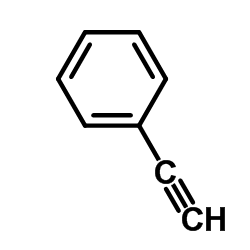

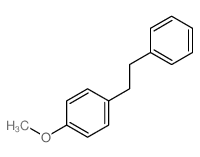
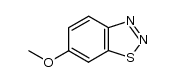

![苯并[d] [1,2,3]噻二唑结构式](https://image.chemsrc.com/caspic/190/273-77-8.png)
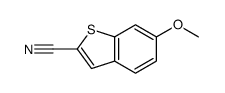
![2-phenylbenzo[b]thiophene结构式](https://image.chemsrc.com/caspic/004/1207-95-0.png)