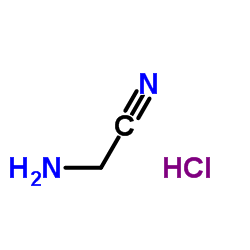
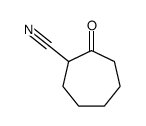
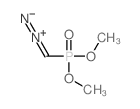
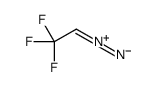
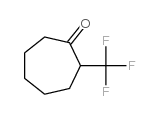
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
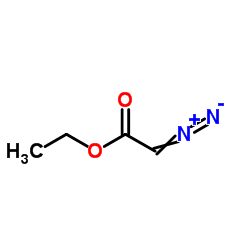
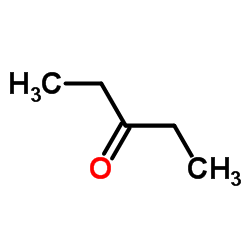
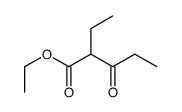


![2-[(3-nitrophenyl)methylidene]cycloheptan-1-one结构式](https://image.chemsrc.com/caspic/032/60719-15-5.png)