|
~87% |
|
~95% |
|
~53% |
|
~7% |
|
~81% |
|
~% |
|
~75% |
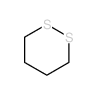

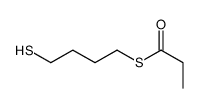
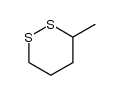
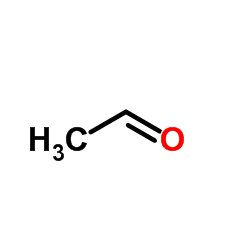

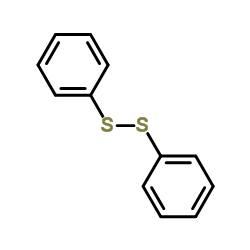
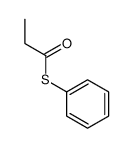

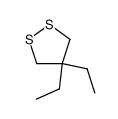
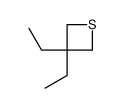
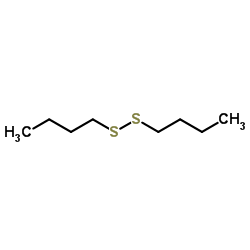
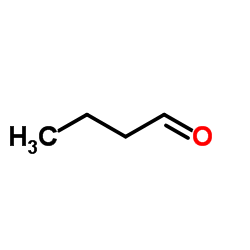
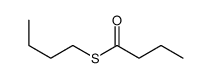

![S-[2-ethyl-2-(sulfanylmethyl)butyl] ethanethioate结构式](https://image.chemsrc.com/caspic/302/59416-44-3.png)