| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
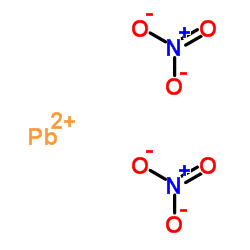 |
硝酸铅
CAS:10099-74-8 |
|
 |
硫酸亚铁分析滴定液
CAS:7782-63-0 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
硝酸铅
CAS:10099-74-8 |
|
 |
硫酸亚铁分析滴定液
CAS:7782-63-0 |