Asymmetric reduction of o-chloroacetophenone with Candida pseudotropicalis 104.
Qing Xie, Jianping Wu, Gang Xu, Lirong Yang
文献索引:Biotechnol. Prog. 22(5) , 1301-4, (2006)
全文:HTML全文
摘要
The asymmetric reduction of o-chloroacetophenone 1 with Candida pseudotropicalis 104 produced the corresponding (S)-1-(2-chloro-phenyl)-ethanol 2 with the enantiomeric excess (ee >99%) without addition of any cosolvent. The cell could tolerate high ketone 1 concentration of 233.8 mmol/L (i.e., 36 g/L) with considerable reduction activity in this method. The product 2 concentration achieved 38.9 and 58.4 mmol/L with cells of 40 and 60 g(DCW) (dry cell weight)/L, respectively, in 24 h. The optimum reaction time, the effect of substrate concentration, cosubstrate type and concentration, and cell concentration in the reaction were investigated in this paper.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
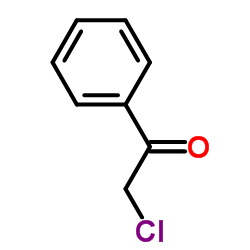 |
2-氯苯乙酮
CAS:532-27-4 |
C8H7ClO |
|
2-Pyridinealdoxime, a new ligand for a Pd-precatalyst: appli...
2005-01-01 [Mol. Divers. 9(4) , 333-9, (2005)] |
|
The use of chemical incapacitant sprays: a review.
2002-03-01 [J. Trauma 52(3) , 595-600, (2002)] |
|
Occupational contact dermatitis due to 2-chloracetophenone t...
1999-03-01 [Br. J. Dermatol. 140(3) , 531-4, (1999)] |
|
Effect of food seasoning spices mixture on biomarkers of oxi...
2007-03-01 [J. Med. Food 10(1) , 149-53, (2007)] |
|
Thermal decomposition studies of riot control agent ω-chloro...
2010-12-15 [J. Hazard. Mater. 184(1-3) , 506-14, (2010)] |