Protein phosphatase 1-dependent dephosphorylation of Akt is the prime signaling event in sphingosine-induced apoptosis in Jurkat cells.
Faisal Thayyullathil, Shahanas Chathoth, Allen Shahin, Jaleel Kizhakkayil, Abdulkader Hago, Mahendra Patel, Sehamuddin Galadari
文献索引:J. Cell. Biochem. 112(4) , 1138-53, (2011)
全文:HTML全文
摘要
Sphingosine (SPH) is an important bioactive lipid involved in mediating a variety of cell functions including apoptosis. However, the signaling mechanism of SPH-induced apoptosis remains unclear. We have investigated whether SPH inhibits survival signaling in cells by inhibiting Akt kinase activity. This study demonstrates that treatment of Jurkat cells with SPH leads to Akt dephosphorylation as early as 15 min, and the cells undergo apoptosis after 6 h. This Akt dephosphorylation is not mediated through deactivation of upstream kinases, since SPH does not inhibit the upstream phosphoinositide-dependent kinase 1 (PDK1) phosphorylation. Rather, sensitivity to the Ser/Thr protein phosphatase inhibitors (calyculin A, phosphatidic acid, tautomycin, and okadaic acid) indicates an important role for protein phosphatase 1 (PP1) in this process. In vitro phosphatase assay, using Akt immunoprecipitate following treatment with SPH, reveals an increase in Akt-PP1 association as determined by immunoprecipitation analysis. Moreover, SPH-induced dephosphorylation of Akt at Ser(473) subsequently leads to the activation of GSK-3β, caspase 3, PARP cleavage, and ultimately apoptosis. Pre-treatment with caspase 3 inhibitor z-VAD-fmk and Ser/Thr phosphatase inhibitor abrogates the effect of SPH on facilitating apoptosis. Altogether, these results demonstrate that PP1-mediated inhibition of the key anti-apoptotic protein, Akt, plays an important role in SPH-mediated apoptosis in Jurkat cells.Copyright © 2011 Wiley-Liss, Inc.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
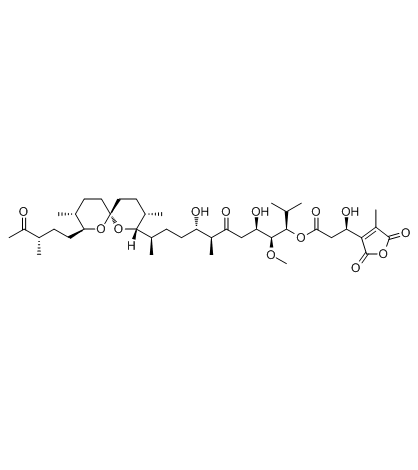 |
互变霉素
CAS:109946-35-2 |
C41H66O13 |
|
Characterization of the tautomycetin biosynthetic gene clust...
2009-03-27 [J. Nat. Prod. 72(3) , 450-9, (2009)] |
|
The bifunctional glyceryl transferase/phosphatase OzmB belon...
2006-08-16 [J. Am. Chem. Soc. 128(32) , 10386-7, (2006)] |
|
PR55 alpha, a regulatory subunit of PP2A, specifically regul...
2009-08-21 [J. Biol. Chem. 284 , 22649 - 22656, (2009)] |
|
Tautomycin suppresses growth and neuroendocrine hormone mark...
2009-01-01 [Am. J. Surg. 197(3) , 313-9, (2009)] |
|
Tautomycin's interactions with protein phosphatase 1.
2010-03-01 [Chem. Asian J. 5(3) , 410-20, (2010)] |