| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
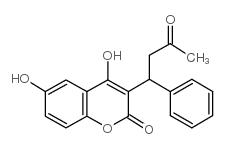 |
6-羟基华法林
CAS:17834-02-5 |
|
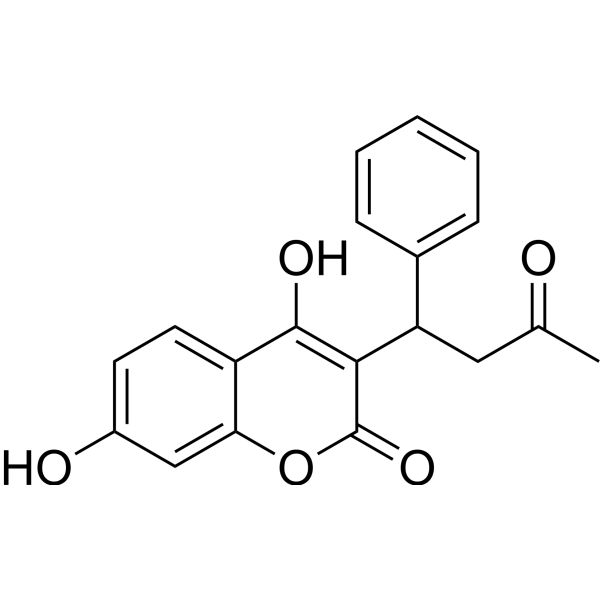 |
7-羟基华法林
CAS:17834-03-6 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
6-羟基华法林
CAS:17834-02-5 |
|
 |
7-羟基华法林
CAS:17834-03-6 |