Fluorescence studies on the interactions of barbaloin with bovine serum albumin.
Jianniao Tian, Jiaqin Liu, Jiyou Zhang, Zhide Hu, Xinguo Chen
文献索引:Chem. Pharm. Bull. 51(5) , 579-82, (2003)
全文:HTML全文
摘要
The fluorescence quenching reactions of barbaloin with bovine serum albumin (BSA) in pH 7.20 Tris-HCl buffer solution were studied. The quenching mechanism of BSA by barbaloin was interpreted using the Stern-Volmer (S-V) mechanism. The binding constant K values were 2.78 x 10(5) (293 K), 1.87 x 10(5) (310 K), 1.25 x 10(5) (318 K), and the number of binding sites (n) were 1.18, 1.14, and 1.09, respectively. In addition, the thermodynamic functions enthalpy (deltaH degrees ) and entropy (deltaS degrees ) for the reaction were also calculated according to Vant's Hoff equation were -23.7 kJ/mol and 23.6 J/mol, respectively. Plausible explanations of the quenching mechanism are discussed on the basis of a hydrophobic interaction between barbaloin and BSA.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
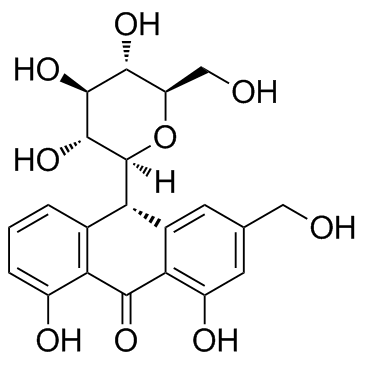 |
芦荟苷
CAS:1415-73-2 |
C21H22O9 |
|
The content of secondary phenol metabolites in pruned leaves...
2008-10-01 [J. Nat. Med. 62(4) , 430-5, (2008)] |
|
Determination of aloenin, barbaloin and isobarbaloin in aloe...
2001-03-05 [J. Chromatogr. B. Biomed. Sci. Appl. 752(1) , 91-7, (2001)] |
|
Liquid chromatographic determination of barbaloin (aloin) in...
1985-01-01 [J. Assoc. Off. Anal. Chem. 68(3) , 493-4, (1985)] |
|
Isolation of a human intestinal bacterium capable of transfo...
1991-02-01 [Planta Med. 57(1) , 15-9, (1991)] |
|
Metabolism of barbaloin by intestinal bacteria.
1988-11-01 [Chem. Pharm. Bull. 36(11) , 4462-6, (1988)] |