| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
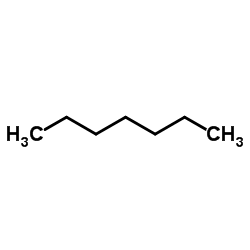 |
正庚烷
CAS:142-82-5 |
|
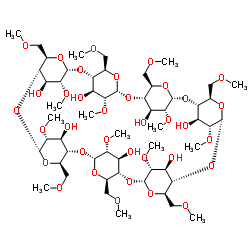 |
2,6-二-O-甲基-β-环糊精
CAS:51166-71-3 |
|
 |
富马酸氯马斯汀
CAS:14976-57-9 |