Sequential and specific exchange of multiple coiled-coil components.
Nathan A Schnarr, Alan J Kennan
文献索引:J. Am. Chem. Soc. 125(43) , 13046-51, (2003)
全文:HTML全文
摘要
The capacity for sequential and specific exchange of single peptides from coiled-coil heterotrimers is investigated. Dual hydrophobic-hydrophilic interface systems permit iterative cycles of pH-triggered strand exchange that can specifically replace one, two, or even all three initial trimer components. The resultant new complexes are either resistant to or capable of further exchange. Control experiments demonstrate that background exchange among different complexes is negligible. When triggered, however, selective displacement of the same peptide from only one of two distinct heterotrimers is feasible. Previously documented peptidic cross-linking strategies remain operative in these more intricate environments.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
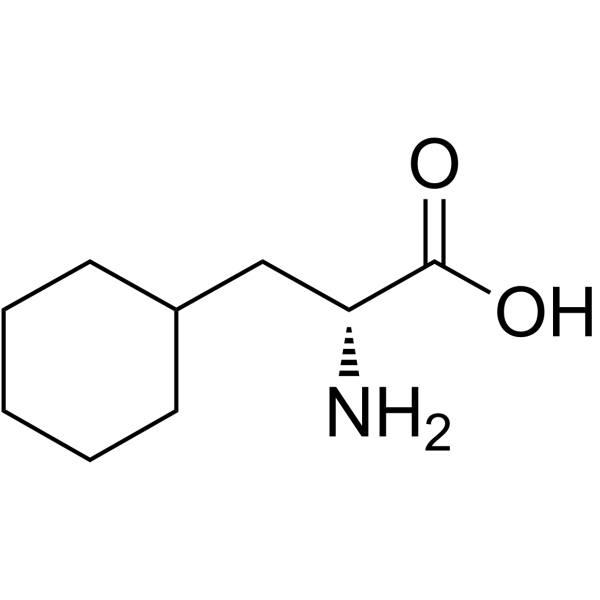 |
D-环己基丙氨酸
CAS:58717-02-5 |
C9H17NO2 |
|
Experimental evaluation of CH-π interactions in a protein co...
2012-05-07 [Chemistry 18(19) , 5832-6, (2012)] |
|
Mapping the ligand-binding site on the C5a receptor: arginin...
2001-11-20 [Biochemistry 40(46) , 14047-52, (2001)] |
|
Synthesis and characterization of more potent analogues of h...
1998-09-29 [Biochemistry 37(39) , 13507-15, (1998)] |
|
Up-regulation of adenosine A1 receptor binding in pentylenet...
2005-01-25 [Brain Res. 1032(1-2) , 94-103, (2005)] |
|
Expression levels of adenosine receptors in hippocampus and ...
2007-08-23 [Neurosci. Lett. 423(3) , 194-9, (2007)] |