Journal of Natural Products
2007-07-01
Microbial transformation of 20(S)-protopanaxatriol-type saponins by Absidia coerulea.
Guangtong Chen, Min Yang, Zhiqiang Lu, Jinqiang Zhang, Huilian Huang, Yan Liang, Shuhong Guan, Yan Song, Lijun Wu, De-an Guo
文献索引:J. Nat. Prod. 70(7) , 1203-6, (2007)
全文:HTML全文
摘要
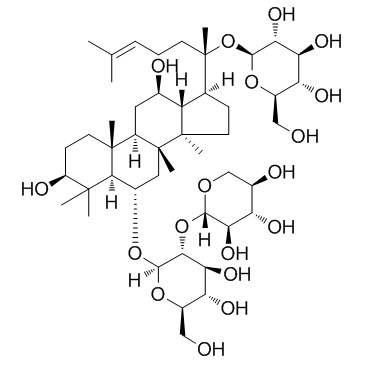
Three 20(S)-protopanaxatriol-type saponins, ginsenoside-Rg1 (1), notoginsenoside-R1 (2), and ginsenoside-Re (3), were transformed by the fungus Absidia coerulea (AS 3.3389). Compound 1 was converted into five metabolites, ginsenoside-Rh4 (4), 3beta,2beta,25-trihydroxydammar-(E)-20(22)-ene-6-O-beta-D-glucopyranoside (5), 20(S)-ginsenoside-Rh1 (6), 20(R)-ginsenoside-Rh1 (7), and a mixture of 25-hydroxy-20(S)-ginsenoside-Rh1 and its C-20(R) epimer (8). Compound 2 was converted into 10 metabolites, 20(S)-notoginsenoside-R2 (9), 20(R)-notoginsenoside-R2 (10), 3beta,12beta,25-trihydroxydammar-(E)-20(22)-ene-6-O-beta-D-xylopyranosyl-(1-->2)-beta-D-glucopyranoside (11), 3beta,12beta-dihydroxydammar-(E)-20(22),24-diene-6-O-beta-D-xylopyranosyl-(1-->2)-beta-D-glucopyranoside (12), 3beta,12beta,20,25-tetrahydroxydammaran-6-O-beta-D-xylopyranosyl-(1-->2)-beta-D-glucopyranoside (13), and compounds 4-8. Compound 3 was metabolized to 20(S)-ginsenoside-Rg2 (14), 20(R)-ginsenoside-Rg2 (15), 3beta,12beta,25-trihydroxydammar-(E)-20(22)-ene-6-O-alpha-L-rhamnopyranosyl-(1-->2)-beta-D-glucopyranoside (16), 3beta,12beta-dihydroxydammar-(E)-20(22),24-diene-6-O-alpha-L-rhamnopyranosyl-(1-->2)-beta-D-glucopyranoside (17), 3beta,12beta,20,25-tetrahydroxydammaran-6-O-alpha-L-rhamnopyranosyl-(1-->2)-beta-D-glucopyranoside (18), and compounds 4-8. The structures of five new metabolites, 10-13 and 16, were established by spectroscopic methods.