| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
L-尼古丁
CAS:54-11-5 |
|
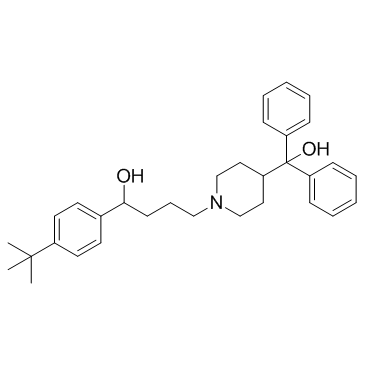 |
特非那定
CAS:50679-08-8 |
|
![8-氯-11-(1-哌嗪基)-5H-二苯并[B,E] [1,4]吡喃 结构式](https://image.chemsrc.com/caspic/371/6104-71-8.png) |
8-氯-11-(1-哌嗪基)-5H-二苯并[B,E] [1,4]吡喃
CAS:6104-71-8 |
|
 |
酮康唑
CAS:65277-42-1 |
|
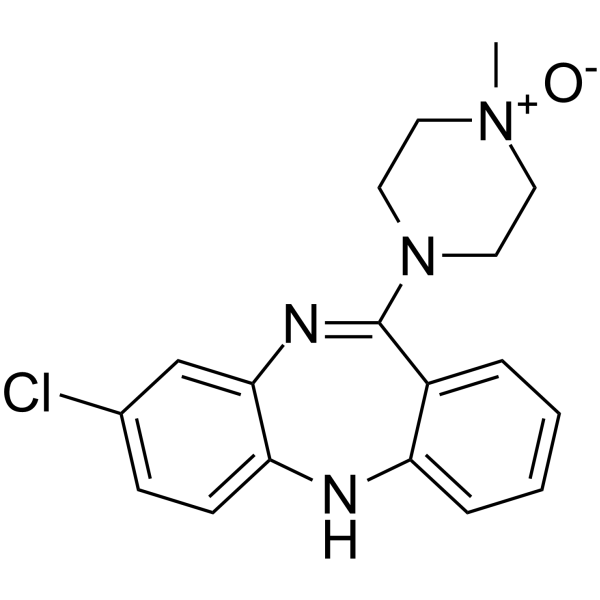 |
氯氮平N-氧化物
CAS:34233-69-7 |