Chemical synthesis of 2'-deoxyoligonucleotides containing 5-fluoro-2'-deoxycytidine.
S Schmidt, C D Pein, H J Fritz, D Cech
文献索引:Nucleic Acids Res. 20(10) , 2421-6, (1992)
全文:HTML全文
摘要
2'-Deoxyoligonucleotides with 5-fluorocytosine residues incorporated at specific positions of the nucleotide sequence are tools of great potential in the study of the catalytic mechanism by which DNA cytosine methyltransferases methylate the 5-position of DNA cytosine residues in specific sequence contexts. Chemical synthesis of such oligonucleotides is described. Two alternative approaches have been developed, one of which proceeds via a fully protected phosphoramidite of 5-fluoro-4-methylmercapto-2'-deoxyuridine 2, the other via a fully protected phosphoramidite of 5-fluoro-2'-deoxycytidine 3. Either building block can be used in automated oligonucleotide synthesis applying standard elongation cycles and deprotection procedures exclusively. The methylmercapto function of 2 is replaced by an amino group in the final ammonia treatment used for cleavage from support and base deprotection.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
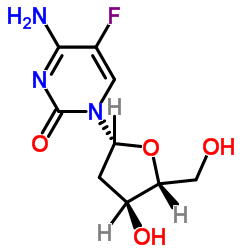 |
5-氟脱氧胞苷
CAS:10356-76-0 |
C9H12FN3O4 |
|
Concentrations of the DNA methyltransferase inhibitor 5-fluo...
2008-07-01 [Cancer Chemother. Pharmacol. 62(2) , 363-8, (2008)] |
|
Five-chlorodeoxycytidine and biomodulators of its metabolism...
1995-07-15 [Int. J. Radiat. Oncol. Biol. Phys. 32(4) , 1059-69, (1995)] |
|
Micronuclei induced by modulators of methylation: analogs of...
1995-07-01 [Carcinogenesis 16(7) , 1647-50, (1995)] |
|
Crystallization and preliminary crystallographic analysis of...
1994-05-13 [J. Mol. Biol. 238(4) , 626-9, (1994)] |
|
Transition state analogs as affinity labels for human DNA me...
1993-10-29 [Biochem. Biophys. Res. Commun. 196(2) , 864-71, (1993)] |