| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
三磷酸腺苷双磷酸酶 来源于马铃薯
CAS:9000-95-7 |
|
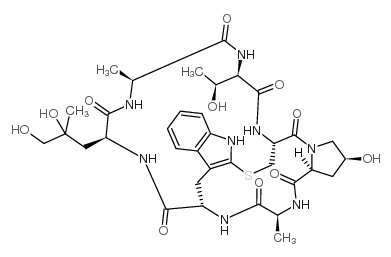 |
鬼笔环肽
CAS:17466-45-4 |