| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
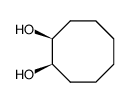 |
顺-1,2-环辛二醇
CAS:27607-33-6 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
顺-1,2-环辛二醇
CAS:27607-33-6 |