Organic Letters
2006-06-08
Total synthesis of (-)-8-O-methyltetrangomycin (MM 47755).
Christian Kesenheimer, Ulrich Groth
文献索引:Org. Lett. 8(12) , 2507-10, (2006)
全文:HTML全文
摘要
A stereoselective total synthesis of the natural antibiotic (-)-8-O-methyltetrangomycin 1 is reported. The essential steps for this convergent synthesis are the transformation of a geraniol epoxide into a chiral octadiyne derivative, which was converted into a triyne. The cobalt-mediated [2+2+2] cycloaddition of the triyne led to a benz[a]anthracene system, which was oxidized with Ag(Py)(2)MnO(4) to a benz[a]anthraquinone. Deprotection with aqueous HF in acetonitrile and photooxidation afforded the desired product (-)-1. [reaction: see text]
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
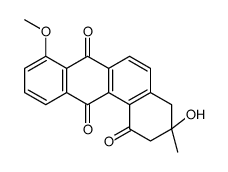 |
MM 47755
CAS:117620-87-8 |
C20H16O5 |
相关文献:
更多...
|
Angucyclinone antibiotics: total syntheses of YM-181741, (+)...
2007-08-03 [J. Org. Chem. 72(16) , 6116-26, (2007)] |
|
The cobalt way to angucyclinones: asymmetric total synthesis...
2010-08-02 [Chemistry 16(29) , 8805-21, (2010)] |
|
Biological activities of deoxyspergualin in autoimmune disea...
1988-09-01 [J. Antibiot. 41(9) , 1253-9, (1988)] |
|
Search method for inhibitors of Staphyloxanthin production b...
2012-01-01 [Biol. Pharm. Bull. 35(1) , 48-53, (2012)] |
|
MM 47755, a new benz[a]anthracene antibiotic from a streptom...
1989-04-01 [J. Antibiot. 42(4) , 627-8, (1989)] |