| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
沙丁胺醇
CAS:18559-94-9 |
|
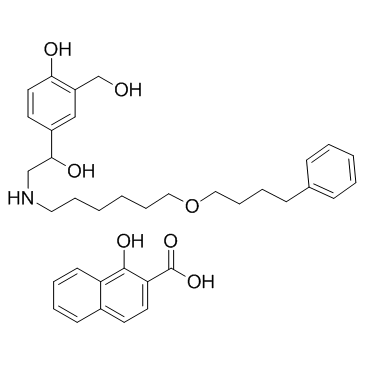 |
昔美酸沙美特罗
CAS:94749-08-3 |
|
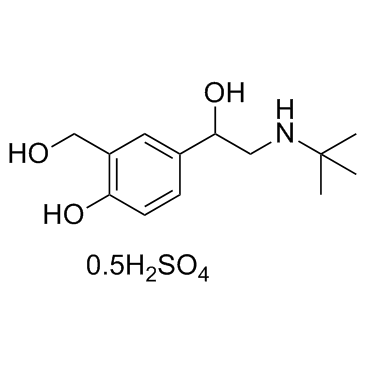 |
硫酸沙丁胺醇
CAS:51022-70-9 |
|
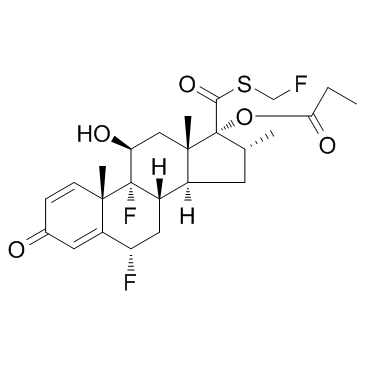 |
氟替卡松丙酸酯
CAS:80474-14-2 |