Synthesis of (-)-PNU-286607 by asymmetric cyclization of alkylidene barbiturates.
J Craig Ruble, Alexander R Hurd, Timothy A Johnson, Debra A Sherry, Michael R Barbachyn, Peter L Toogood, Gordon L Bundy, David R Graber, Gregg M Kamilar
文献索引:J. Am. Chem. Soc. 131(11) , 3991-7, (2009)
全文:HTML全文
摘要
PNU-286607 is the first member of a promising, novel class of antibacterial agents that act by inhibiting bacterial DNA gyrase, a target of clinical significance. Importantly, PNU-286607 displays little cross-resistance with marketed antibacterial agents and is active against methicillin-resistant staphylococcus aureus (MRSA) and fluoroquinoline-resistant bacterial strains. Despite the apparent stereochemical complexity of this unique spirocyclic barbituric acid compound, the racemic core is accessible by a two-step route employing a relatively obscure rearrangement of vinyl anilines, known in the literature as the "tert-amino effect." After a full investigation of the stereochemical course of the racemic reaction, starting with the meso cis-dimethylmorpholine, a practical asymmetric variant of this process was developed.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
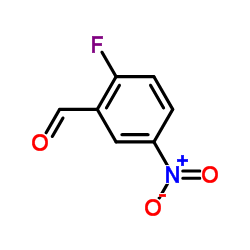 |
2-氟-5-硝基苯甲醛
CAS:27996-87-8 |
C7H4FNO3 |
|
The preparation of some polymethine astrazon dyes. Gale ...
[Aust. J. Chem. 23(5) , 1063-68, (1970)] |
|
Novel synthesis of dibenzo [b,g] 1, 5-oxazocines. Ou...
[Tetrahedron Lett. 40(32) , 5827-30, (1999)] |
|
Facile solid phase synthesis of 1, 2-disubstituted-6-nitro-1...
[Tetrahedron Lett. 46(3) , 427-30, (2005)] |
|
A valuable heterocyclic ring transformation: from isoxazolin...
[Tetrahedron 59(50) , 9887-93, (2003)] |