| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
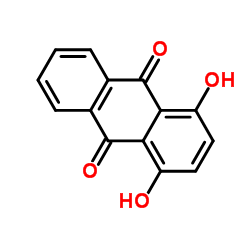 |
1,4-二羟基蒽醌
CAS:81-64-1 |
|
 |
脱氢乙酸钠盐一水合物
CAS:64039-28-7 |
|
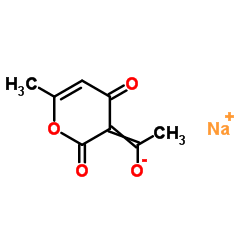 |
脱氢醋酸钠
CAS:4418-26-2 |
|
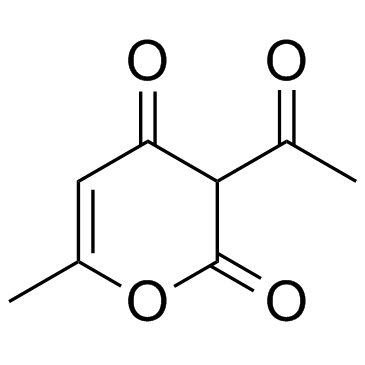 |
脱氢乙酸
CAS:520-45-6 |