| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
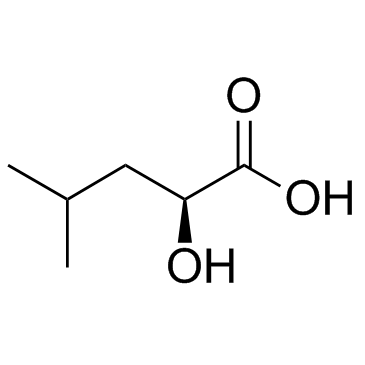 |
(S)-2-羟基-4-甲基戊酸
CAS:13748-90-8 |
|
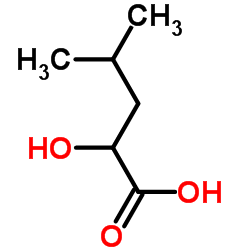 |
DL-闪白酸
CAS:498-36-2 |