3-(7-Azaindolyl)-4-arylmaleimides as potent, selective inhibitors of glycogen synthase kinase-3.
Han-Cheng Zhang, Hong Ye, Bruce R Conway, Claudia K Derian, Michael F Addo, Gee-Hong Kuo, Leonard R Hecker, Diane R Croll, Jian Li, Lori Westover, Jun Z Xu, Richard Look, Keith T Demarest, Patricia Andrade-Gordon, Bruce P Damiano, Bruce E Maryanoff
文献索引:Bioorg. Med. Chem. Lett. 14 , 3245-3250, (2004)
全文:HTML全文
摘要
A novel series of acyclic 3-(7-azaindolyl)-4-(aryl/heteroaryl)maleimides was synthesized and evaluated for activity against GSK-3beta and selectivity versus PKC-betaII, as well as a broad panel of protein kinases. Compounds 14 and 17c potently inhibited GSK-3beta (IC(50)=7 and 26 nM, respectively) and exhibited excellent selectivity over PKC-betaII (325 and >385-fold, respectively). Compound 17c was also highly selective against 68 other protein kinases. In a cell-based functional assay, both 14 and 17c effectively increased glycogen synthase activity by inhibiting GSK-3beta.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
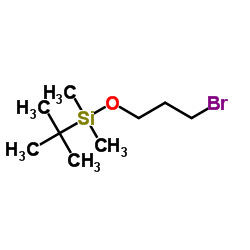 |
3-溴丙基叔丁基二甲基硅醚
CAS:89031-84-5 |
C9H21BrOSi |
|
Synthesis and selective cyclooxygenase-2 inhibitory activity...
2004-04-22 [J. Med. Chem. 47 , 2180-2193, (2004)] |
|
A concise total synthesis of (+/-)-vigulariol.
2007-01-01 [Angew. Chem. Int. Ed. Engl. 46 , 437, (2007)] |
|
Synthetic approaches to indolo[6,7-a]pyrrolo[3,4-c]carbazole...
2004-04-30 [J. Org. Chem. 69 , 2967-2975, (2004)] |
|
Synthesis, radioiodination and in vivo screening of n...
[European J. Org. Chem. 63 , 840-853, (2013)] |
|
Synthesis of O-(3-[18F] Fluoropropyl)-L-ty...
[Bull. Korean Chem. Soc. 26(1) , 91-96, (2005)] |