A single-step procedure for the synthesis of photoreactive and radioactive glycerolipids.
Suman Lata, Kanchan Bhardwaj, Ram Rajasekharan
文献索引:Anal. Biochem. 313(1) , 155-9, (2003)
全文:HTML全文
摘要
A single-step procedure was developed for the incorporation of iodoazide into oleic acid, triolein, and phosphatidylcholine. Iodoazide was generated using [125I]iodomonochloride and sodium azide that was found to add stereospecifically in a variety of olefins. Photoreactive and radiolabeled triacylglycerol and phosphatidylcholine were synthesized with a moderate yield and high specific activity. The stability of both the radiolabel and the photoreactive group was studied under ultraviolet light under aqueous as well as anhydrous conditions. These synthesized analogs act as substrates in the dark, and as irreversible inhibitors under ultraviolet irradiation for the target hydrolytic enzymes. The synthesized radiolabeled photoprobes were subsequently used to label lipase and phospholipase A(2). The results highlighted the efficiency and rapidity of the method and its potential application in the study of lipid-metabolizing enzymes.Copyright 2003 Elsevier Science (USA)
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
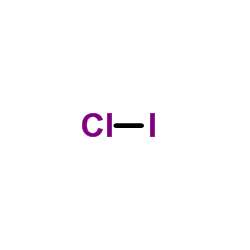 |
一氯化碘
CAS:7790-99-0 |
ClI |
|
Self-Assembly of a Tripod Aromatic Rod into Stacked Planar N...
2015-08-10 [Chemistry 21 , 11836-42, (2015)] |
|
Cell surface labeling by iodine monochloride.
1981-01-01 [Int. J. Biochem. 13(9) , 1011-7, (1981)] |
|
Rapid extraction, radioiodination, and in vivo catabolism of...
1985-12-01 [Am. J. Vet. Res. 46(12) , 2578-81, (1985)] |
|
Position of gamma-chain carboxy-terminal regions in fibrinog...
2000-11-21 [Biochemistry 39(46) , 14171-5, (2000)] |
|
Iodinated soluble adult worm protein preparations of Schisto...
1991-01-01 [Braz. J. Med. Biol. Res. 24(8) , 787-90, (1991)] |