| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
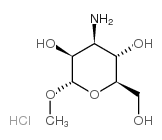 |
3-氨基-3-脱氧-alpha-d-甘露糖苷甲酯盐酸盐
CAS:14133-35-8 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
3-氨基-3-脱氧-alpha-d-甘露糖苷甲酯盐酸盐
CAS:14133-35-8 |