| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
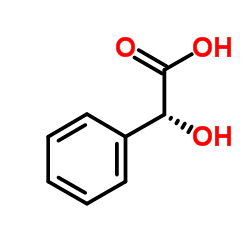 |
(S)-(+)-扁桃酸
CAS:17199-29-0 |
|
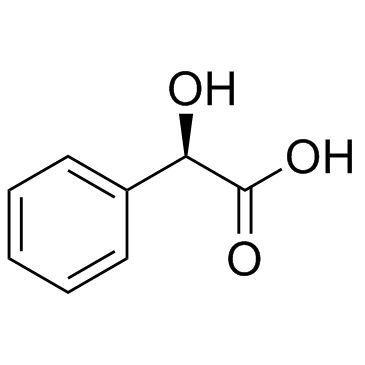 |
扁桃酸
CAS:611-71-2 |
|
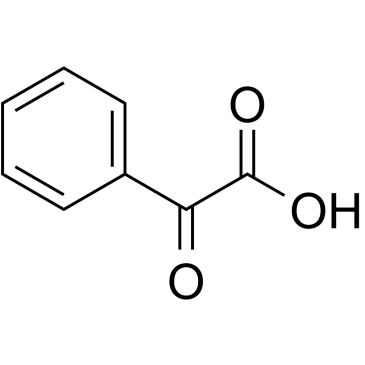 |
苯甲酰甲酸
CAS:611-73-4 |
|
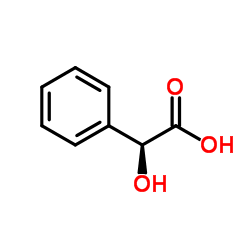 |
DL-扁桃酸
CAS:90-64-2 |