Charge transfer interaction of 4-acetamidophenol (paracetamol) with 2,3-dichloro-1,4-naphthoquinone: a study in aqueous ethanol medium by UV-vis spectroscopic and DFT methods.
Avijit Saha, Amit S Tiwary, Asok K Mukherjee
文献索引:Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 71(3) , 835-40, (2008)
全文:HTML全文
摘要
4-Acetamidophenol (paracetamol) is shown to form charge transfer complex with 2,3-dichloro1,4-naphthoquinone in aqueous ethanol media exhibiting the unusual 2:1 (paracetamol:quinone) stoichiometry. The complexation enthalpy and entropy have been estimated from the formation constant (K) determined spectrophotometrically at five different temperatures. In aqueous ethanol mixtures of varying composition K increases with increasing dielectric constant of the medium. This has been rationalized by calculating the electronic charge distribution in paracetamol molecule and its conjugate base at the DFT/B3LYP/6-31++G(d,p) level. The theoretically calculated vertical ionization potential of paracetamol also agrees with reported experimental value.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
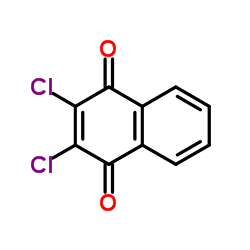 |
2,3-二氯-1,4-萘醌
CAS:117-80-6 |
C10H4Cl2O2 |
|
Indoleamine 2,3-dioxygenase is the anticancer target for a n...
2008-03-27 [J. Med. Chem. 51 , 1706-18, (2008)] |
|
The 1,4-naphthoquinone scaffold in the design of cysteine pr...
2007-08-01 [Bioorg. Med. Chem. 15 , 5340-50, (2007)] |
|
Modulation of hepatic cytochrome P-450 and DT-diaphorase by ...
1988-08-01 [Bull. Environ. Contam. Toxicol. 41(2) , 164-71, (1988)] |
|
Spectroscopic and theoretical studies on the nucleophilic su...
2012-12-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 98 , 378-83, (2012)] |
|
Study of a reaction between 2,3-dichloro-1,4-naphthoquinone ...
1993-01-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 60(7) , 1641-7, (2004)] |