| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
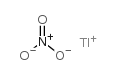 |
硝酸铊
CAS:10102-45-1 |
|
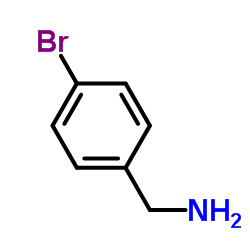 |
4-溴苯甲胺
CAS:3959-07-7 |
|
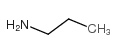 |
丙胺
CAS:107-10-8 |
|
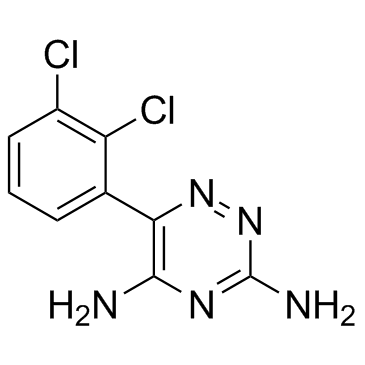 |
拉莫三嗪
CAS:84057-84-1 |
|
 |
利多卡因
CAS:137-58-6 |
|
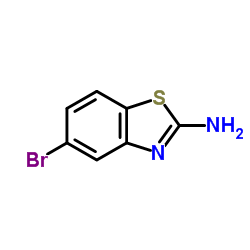 |
2-氨基-5-溴苯并噻唑
CAS:20358-03-6 |
|
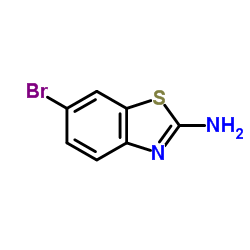 |
2-氨基-6-溴苯并噻唑
CAS:15864-32-1 |