LC method for the quantitative determination of oxaprozin and its impurities in the bulk drug.
K V Reddy, D S Rao, K Vyas, G O Reddy
文献索引:J. Pharm. Biomed. Anal. 22(4) , 651-9, (2000)
全文:HTML全文
摘要
A reversed phase linear gradient liquid chromatographic method was developed for the separation and quantitative determination of the seven known process related impurities and one degraded product of oxaprozin in the bulk drug material. An Inertsil-ODS 3V (150 x 4.6 mm), 5 microm column was operated with a phosphate buffer acetonitrile gradient. Detection was carried out on a UV detector at 254 nm. This method has been proved to be accurate and sensitive. The limits of detection (LOD) and limits of quantification (LOQ) of impurities were in the order of 5-60 ng and 16-200 ng, respectively. In addition to its ruggedness and robustness, this method offers identification of all eight impurities in a single run.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
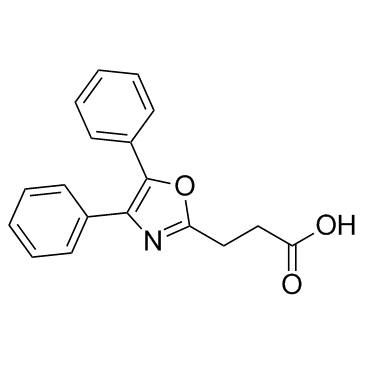 |
奥沙普秦
CAS:21256-18-8 |
C18H15NO3 |
|
Potential therapeutic approach to SAPHO.
2000-04-01 [Semin. Arthritis Rheum. 29(5) , 332-4, (2000)] |
|
Analgesic and Anti-Inflammatory Effects of Oxaprozin and Nap...
2010-01-01 [J. Oral Maxillofac. Surg. 68(5) , 1018-24, (2010)] |
|
Oxaprozin versus diclofenac in NSAID-refractory periarthriti...
2004-08-01 [Curr. Med. Res. Opin. 20(8) , 1279-90, (2004)] |
|
Effects of Transcutol P on the corneal permeability of drugs...
2006-01-01 [J. Pharm. Pharmacol. 58(1) , 45-50, (2006)] |
|
Development of a new delivery system consisting in 'drug-in ...
2010-01-01 [J. Microencapsul. 27(6) , 479-86, (2010)] |