| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
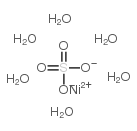 |
硫酸镍(II),六水合物
CAS:10101-97-0 |
|
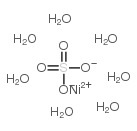 |
硫酸镍七水合物
CAS:10101-98-1 |
|
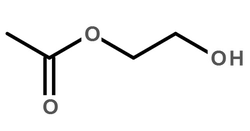 |
羊毛脂
CAS:8006-54-0 |
|
 |
硫酸镍(II)水合物
CAS:15244-37-8 |
|
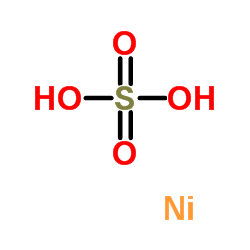 |
硫酸镍
CAS:7786-81-4 |