Pharmacokinetics of valerenic acid after administration of valerian in healthy subjects.
Gail D Anderson, Gary W Elmer, Eric D Kantor, Ian E Templeton, Michael V Vitiello
文献索引:Phytother Res. 19(9) , 801-3, (2005)
全文:HTML全文
摘要
To describe the pharmacokinetics of valerenic acid in a group of healthy adults after a single oral dose of valerian using a newly developed sensitive assay for serum concentrations of valerenic acid, a commonly used marker for qualitative and quantitative analysis of valerian root and valerian products.Six healthy adults (22-61 years, five men, one female) received a single 600 mg dose of valerian at 08:00. Blood samples were collected for 8 h after administration. Valerenic acid was extracted from serum and measured using a LC/MS/MS method developed in our laboratory.The maximum serum concentration of valerenic acid for five of the six subjects occurred between 1 and 2 h ranging from 0.9 to 2.3 ng/mL. Valerenic acid serum concentrations were measurable for at least 5 h after the valerian dose. One subject showed a peak plasma value at 1 h and a second peak at 5 h. The elimination half-life (T(1/2)) for valerenic acid was 1.1 +/- 0.6 h. The area under the concentration time curve (AUC) as a measure of valerenic acid exposure was variable (4.80 +/- 2.96 microg/mL. h) and not correlated with subject's age or weight.Assuming that valerenic acid serum concentrations correlate with the pharmacological activity of valerian, the timing of the valerenic acid peak concentration is consistent with the standard dosage recommendation to take valerian 30 min to 2 h before bedtime. Ongoing studies are evaluating the relationship between valerenic acid serum concentrations and objective measures of sleep in patients.Copyright 2005 John Wiley & Sons, Ltd.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
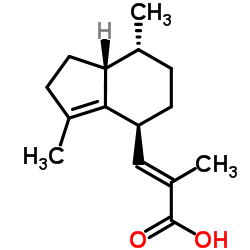 |
缬草素
CAS:3569-10-6 |
C15H22O2 |
|
Concise synthesis of Valerena-4,7(11)-diene, a highly active...
2010-01-01 [Biosci. Biotechnol. Biochem. 74(9) , 1963-4, (2010)] |
|
Root colonization by symbiotic arbuscular mycorrhizal fungi ...
2010-03-01 [Planta Med. 76(4) , 393-8, (2010)] |
|
Valerian extract and valerenic acid are partial agonists of ...
2005-08-18 [Brain Res. Mol. Brain Res. 138(2) , 191-7, (2005)] |
|
Valerian extract characterized by high valerenic acid and lo...
2012-10-15 [Phytomedicine 19(13) , 1216-22, (2012)] |
|
Chemical fingerprinting of valeriana species: simultaneous d...
2006-01-01 [J. AOAC Int. 89(1) , 8-15, (2006)] |