Journal of Organic Chemistry
2010-09-03
Palladium-catalyzed cascade aryl addition/intramolecular lactonization of phthalaldehyde to access 3-aryl- and alkenylphthalides.
Zhishi Ye, Pengcheng Qian, Guanglei Lv, Fang Luo, Jiang Cheng
文献索引:J. Org. Chem. 75(17) , 6043-5, (2010)
全文:HTML全文
摘要
A palladium-catalyzed addition of arylboronic acids to phthalaldehyde, followed by an intramolecular lactonization to access 3-substituted phthalides, is described. The procedure tolerates a series of functional groups, such as methoxyl, fluoro, chloro, and trifluoromethyl groups. It represents a procedure for the synthesis of 3-substituted phthalides.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
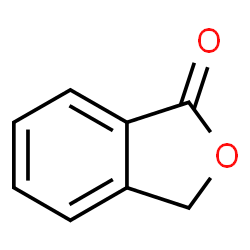 |
苯酞
CAS:87-41-2 |
C8H6O2 |
相关文献:
更多...
|
Development of an Immunosensor for Determination of the Fung...
2015-07-22 [J. Agric. Food Chem. 63 , 6325-30, (2015)] |
|
Design and synthesis of marine fungal phthalide derivatives ...
2012-08-15 [Bioorg. Med. Chem. 20(16) , 4954-61, (2012)] |
|
Phthalide derivatives with antifungal activities against the...
2011-11-01 [J. Antibiot. 64(11) , 723-7, (2011)] |
|
Metabolic profiling of GuanXin II prescription based on meta...
2011-03-25 [J. Pharm. Biomed. Anal. 54(4) , 789-98, (2011)] |
|
Adulticidal activity of phthalides identified in Cnidium off...
2011-08-10 [J. Agric. Food Chem. 59(15) , 8193-8, (2011)] |