Cyclopeptine synthetase activity in surface cultures of Penicillium cyclopium.
W Lerbs, M Luckner
文献索引:J. Basic Microbiol. 25(6) , 387-91, (1985)
全文:HTML全文
摘要
Cyclopeptine synthetase, the key enzyme of benzodiazepine alkaloid biosynthesis in Penicillium cyclopium forms cyclo-(anthranoyl-phenylalanyl) from anthranilic acid, L-phenylalanine, the methyl group of L-methionine and ATP. The following in vitro measurable partial activities of the enzyme system were followed during the development of P. cyclopium: anthranilic acid and L-phenylalanine adenylyltransferase activities, and the ability for thioester-binding of L-phenylalanine to the enzyme protein. These activities became measurable at the beginning of the idiophase and reached a maximum 6 days after inoculation, i.e., the pattern of activity was similar to that of the other enzymes participating in the biosynthesis of the benzodiazepine alkaloids indicating that the activities of all enzymes of the pathway were coordinatedly expressed. Inhibitor experiments indicated that 48-55 h after inoculation a preprotein of anthranilic acid adenylyltransferase was formed, which later on became activated by a hitherto unknown mechanism.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
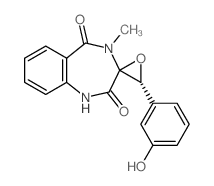 |
环酚
CAS:20007-85-6 |
C17H14N2O4 |
|
Penicillium discolor, a new species from cheese, nuts and ve...
1997-08-01 [Antonie van Leeuwenhoek 72(2) , 119-26, (1997)] |
|
Nuclear inheritance of the biosynthesis of cyclopenin and cy...
1984-01-01 [Z. Allg. Mikrobiol. 24(9) , 615-8, (1984)] |
|
Uric acid is a genuine metabolite of Penicillium cyclopium a...
2002-04-01 [Appl. Environ. Microbiol. 68(4) , 1524-33, (2002)] |
|
PTP1B inhibitory secondary metabolites from marine-derived f...
2013-09-28 [J. Microbiol. Biotechnol. 23(9) , 1206-11, (2013)] |