| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
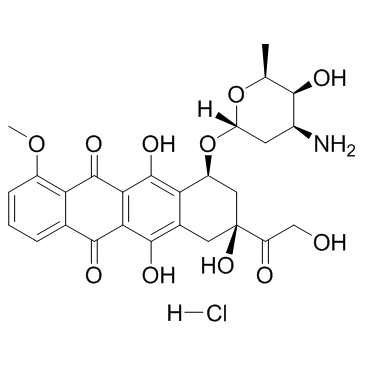 |
盐酸阿霉素
CAS:25316-40-9 |
|
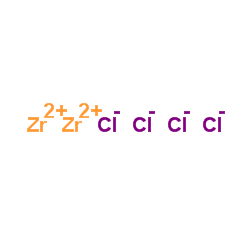 |
四氯化锆
CAS:10026-11-6 |
|
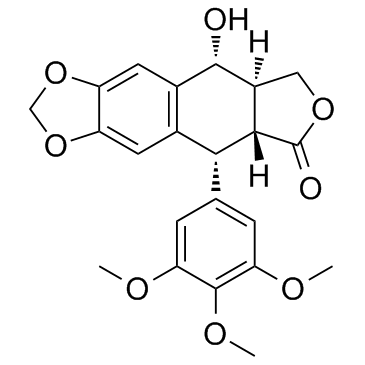 |
鬼臼毒素
CAS:518-28-5 |