Synthesis and dual D2 and 5-HT1A receptor binding affinities of 5-piperidinyl and 5-piperazinyl-1H-benzo[d]imidazol-2(3H)-ones.
Nisar Ullah
文献索引:J. Enzyme Inhib. Med. Chem. , (2013)
全文:HTML全文
摘要
A series of new 5-piperidinyl and 5-piperazinyl-1H-benzo[d]imidazol-2(3H)-ones have been synthesized and evaluated for dual D2 and 5-HT1A receptor binding affinities. The synthesized ligands are structurally related to bifeprunox, a potential atypical antipsychotic, having potent D2 receptor antagonist and 5-HT1A receptor agonist properties. The Suzuki-Miyaura reaction of cyclic vinyl boronate with appropriate aryl halide yielded arylpiperidine, which was eventually transformed to piperidinyl-1H-benzo[d]imidazol-2(3H)-one. The reductive amination of the latter with appropriate biarylaldehdyes rendered the synthesis of 5-piperidinyl-1H-benzo[d]imidazol-2(3H)-ones. Likewise, the Buchwald-Hartwig coupling reactions of 1-boc-piperazine with appropriate aryl halide and subsequent removal of the boc group rendered arylpiperazine. The reductive amination of the latter with appropriate biarylaldehdyes accomplished the synthesis of 5-piperazinyl-1H-benzo[d]imidazol-2(3H)-ones. The structure-activity relationship studies showed that cyclopentenylpyridine and cyclopentenylbenzyl groups contribute significantly to the dual D2 and 5-HT1A receptor binding affinities of these compounds.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
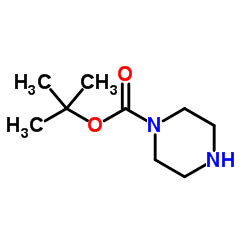 |
1-Boc-哌嗪
CAS:57260-71-6 |
C9H18N2O2 |
|
Design, synthesis and structure-activity relationship studie...
2004-06-07 [Bioorg. Med. Chem. Lett. 14 , 2857-2862, (2004)] |
|
Molecular iodine-catalyzed facile procedure for N-Boc protec...
2006-10-13 [J. Org. Chem. 71 , 8283, (2006)] |
|
Inhibition of noroviruses by piperazine derivatives.
2012-01-01 [Bioorg. Med. Chem. Lett. 22(1) , 377-9, (2012)] |
|
1-alkyl-4-acylpiperazines as a new class of imidazole-free h...
2004-05-20 [J. Med. Chem. 47 , 2833-2838, (2004)] |
|
Efficient copper-catalyzed cross-coupling of 1-Boc-piperazin...
[Tetrahedron Lett. 54(39) , 5332-34, (2013)] |