| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
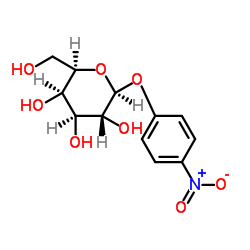 |
对硝基苯基-β-D-吡喃半乳糖苷
CAS:3150-24-1 |
|
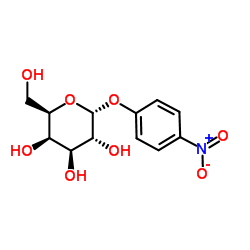 |
4-硝基苯-α-D-吡喃半乳糖苷
CAS:7493-95-0 |