| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
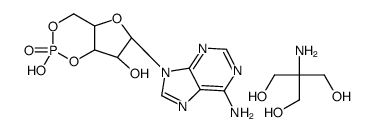 |
腺苷3',5'-环单磷酸三羟甲基氨基甲烷盐
CAS:102029-77-6 |
|
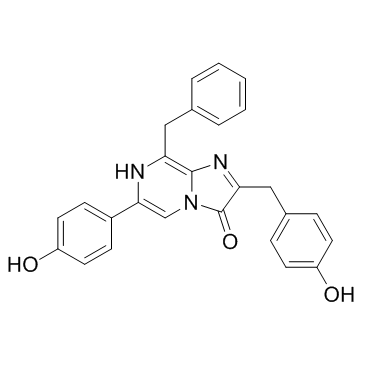 |
腔肠素
CAS:55779-48-1 |
|
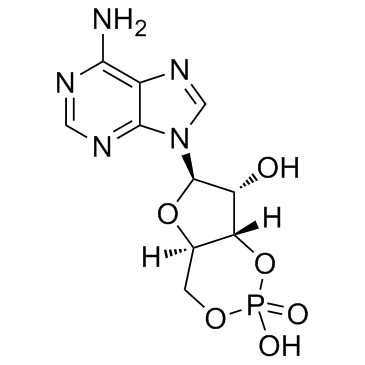 |
环磷酸腺苷
CAS:60-92-4 |